Overview
In a reversal of policy, the U.S. Department of Health and Human Services (HHS) formally abandoned its 2025 340B Rebate Model Pilot Program. This decision follows a series of legal setbacks in federal court and is immediately followed by a new Request for Information (RFI) issued on February 13, 2026.
This alert summarizes the collapse of the initial pilot and the start of a new process that could shape the future of 340B drug pricing.
1. The Quick Rise and Fall of the 2025 Pilot Program
In August 2025, the Health Resources Service Administration (HRSA) announced a voluntary pilot program to transition the 340B benefit from an upfront discount to a backend rebate model.
- Target: Initially limited to 10 high-cost drugs subject to Medicare price negotiation.
- The Model: Safety-net providers (called 340B covered entities) would pay the full Wholesale Acquisition Cost (WAC) and later submit claims to manufacturers for a rebate of the 340B savings.
- The Conflict: Covered entities argued the model created “irreparable harm” through massive cash-flow disruptions and administrative burdens. The American Hospital Association (AHA) sued the federal government to prevent implementation of the rebate model. On December 29, 2025, the U.S. District Court for the District of Maine blocked the program, ruling that HRSA's “threadbare administrative record” failed to consider 30 years of hospital reliance on upfront discounts. On January 7, 2026, the U.S. Court of Appeals for the First Circuit denied the government's request to stay the injunction, agreeing that the government failed to show a likelihood of success on the merits. On February 5, 2026, recognizing the legal flaws, the Department of Justice (DOJ) filed a joint motion to vacate the program entirely and remand it to HRSA, effectively ending the current version of the pilot.
2. HRSA’s New RFI
As of today, February 13, 2026, HRSA has pivoted to a formal information-gathering phase. This RFI signals a “reset” rather than a total abandonment of the rebate concept.
Key areas where HRSA is seeking stakeholder input include:
- Operational Impact: Specifically, the cost of hiring new staff or modifying IT systems to track and claim rebates.
- Cash-Flow Concerns: Evidence regarding whether a rebate model would compromise the ability of safety-net providers to maintain services.
- Guardrails: Suggestions for specific “safeguards” to prevent manufacturers from unfairly denying rebate claims.
- Statutory Interpretation: Whether the 340B statute's language allows for a choice between “rebates” and “discounts” at the agency’s discretion.
Where are we now? Strategic Implications for Stakeholders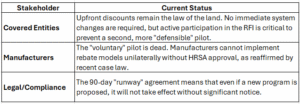
Critical Deadline: Comments on the new HRSA RFI are due by March 19, 2026.
Contact Shumaker Advisors Ryan Walker and Chris Salemme, Shumaker Partner Daphne Kackloudis, or a member of the Shumaker Health Law Team for assistance drafting and filing responses to the RFI.